UNIT ____: Protein Structure and Function Name: _____________________
Essential Idea(s):
Proteins have a very wide range of functions in living organisms.
2.1.S1: Drawing molecular diagrams of glucose, ribose, a saturated fatty acid and a generalized amino acid
- Draw the generalized structure of an amino acid.
- Label the amine group, carboxyl group, alpha carbon and R group on an amino acid.
2.4.U2: There are 20 different amino acids in polypeptides synthesized on ribosomes.
- State the number of amino acids used by living organisms to make polypeptides.
- Given an image of an amino acid, classify the amino acid chemical properties based on R group properties.
2.4.NOS: Looking for patterns, trends, and discrepancies- most but not all organisms assemble proteins from the same amino acids.
- Explain the trend of organisms’ assembly of polypeptides from the same amino acids.
- Describe a discrepancy of the trend of all organisms using the same amino acids to assemble polypeptides.
D.1.U4: Some fatty acids and some amino acids are essential.
- Outline the concept of “conditionally essential” using amino acid examples.
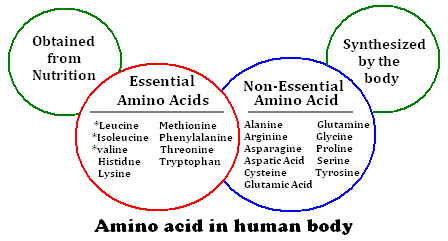
D.1.U1: Essential nutrients cannot be synthesized by the body, therefore they have to be included in the diet.
- Define “essential” as related to dietary nutrients.
- Define “non-essential” as related to dietary nutrients.
D.1.U5: Lack of essential amino acids affects the production of proteins.
- Outline the effect of protein deficiency malnutrition on children and adults.
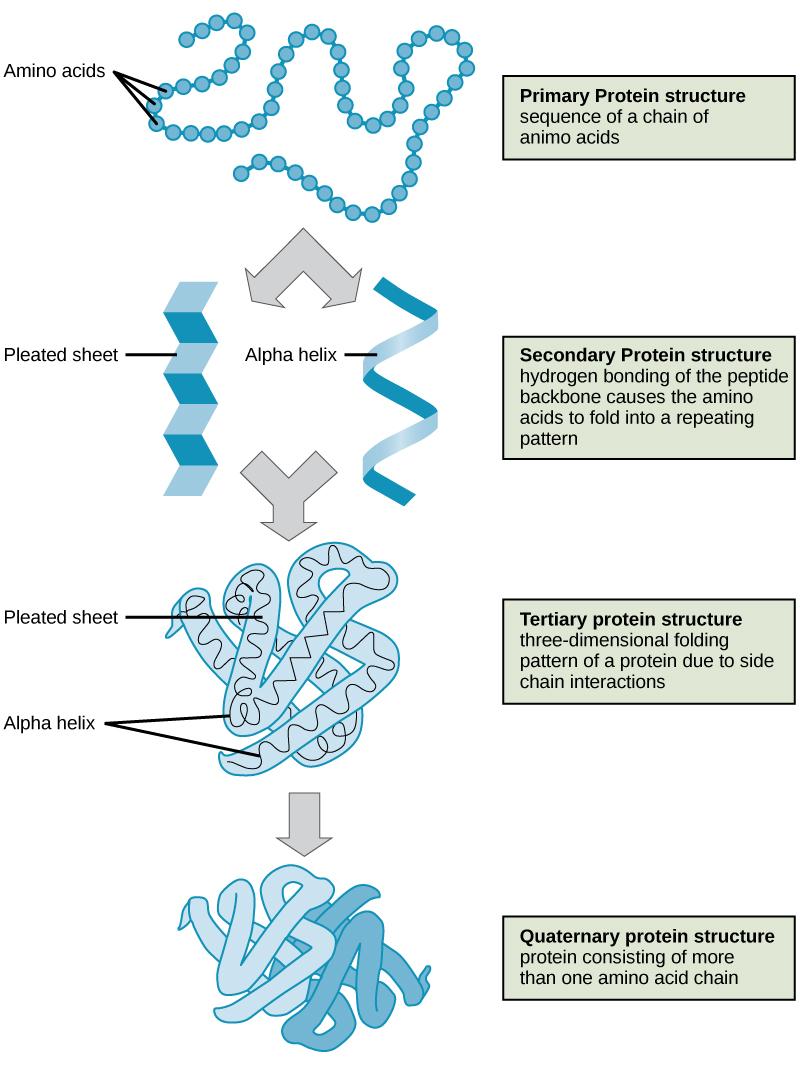
7.3.U7: The sequence and number of amino acids in the polypeptide is the primary structure.
- Describe the primary structure of a protein, including the type of bonding involved.
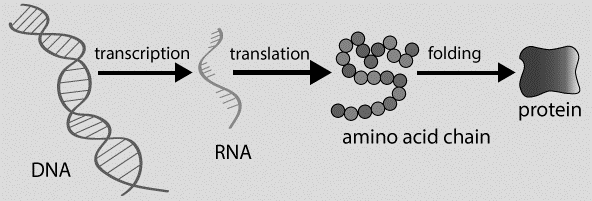
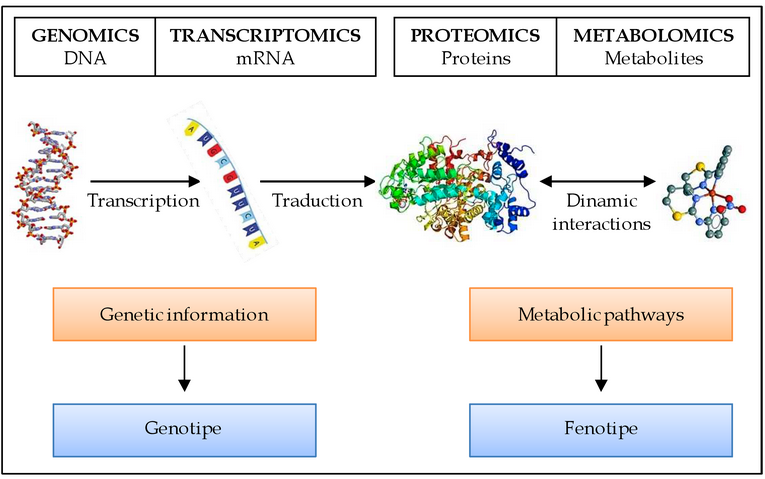
2.4.U4: The amino acid sequence of polypeptides is coded for by genes.
- Outline the relationship between genes and polypeptides.
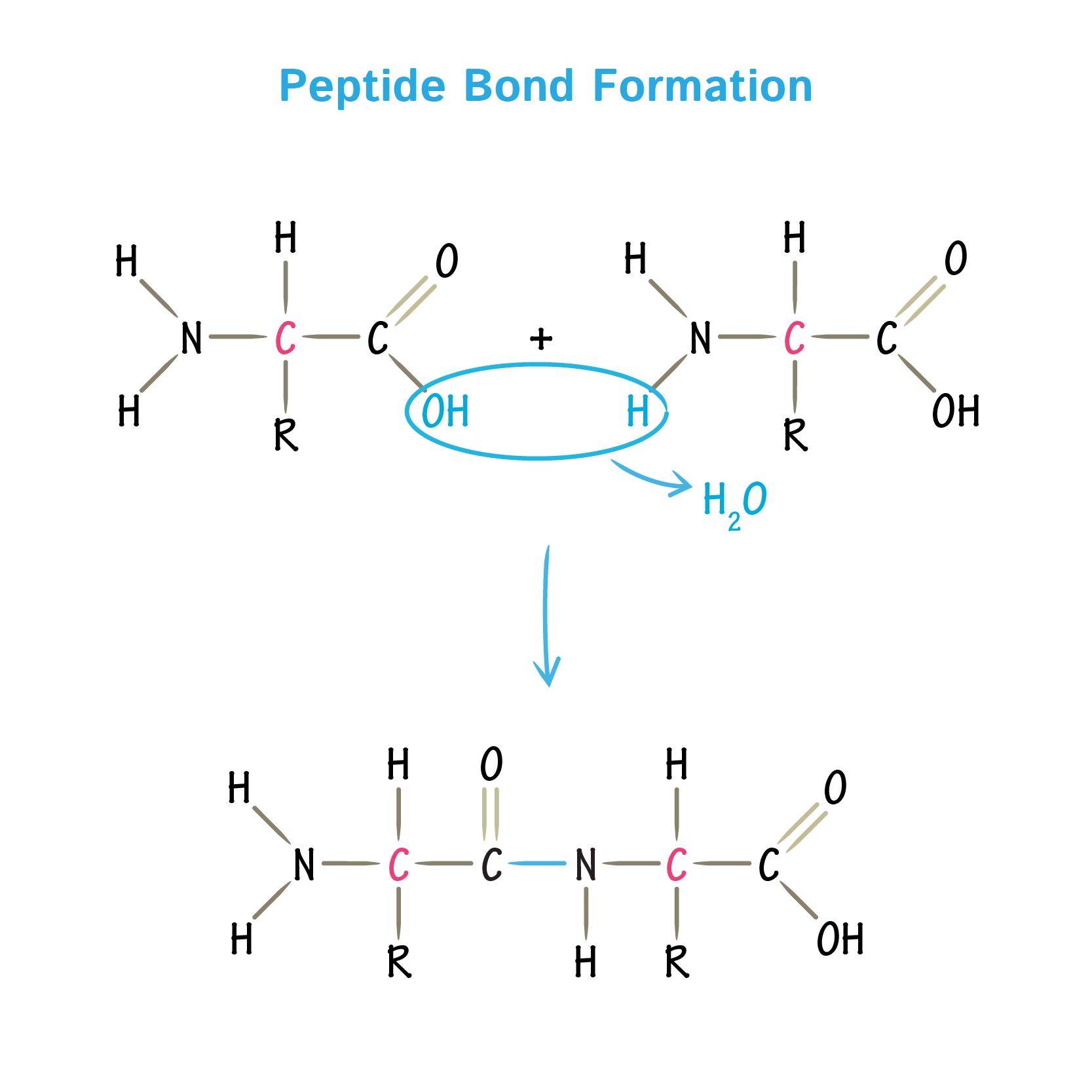
2.4.U1: Amino acids are linked together by condensation to form polypeptides.
- Describe polypeptide chain formation in terms of the formation of peptide bonds and condensation reactions.
2.4.S1: Drawing molecular diagrams to show the formation of a peptide bond.
- Draw peptide bond formation in a condensation reactions.
2.4.U3: Amino Acids can be linked together in any sequence giving a huge range of possible polypeptides.
- Calculate the possible number of amino acid sequences given n number of amino acids.
7.3.U8: The secondary structure is the formation of alpha helices and beta pleated sheets stabilized by hydrogen bonding.
- Describe the secondary structure of a protein, including the type of bonding involved.
- Identify the alpha-helix and beta-pleated sheet in images of protein structure.
7.3.U9: The tertiary structure is the further folding of the polypeptide stabilized by interactions between R groups.
- Describe the tertiary structure of a protein, including the types of R group interactions involved.
- Explain how the chemical characteristics of R groups in the polypeptide chain affect protein folding.
2.4.U6: The amino acid sequence determines the three-dimensional conformation of a protein.
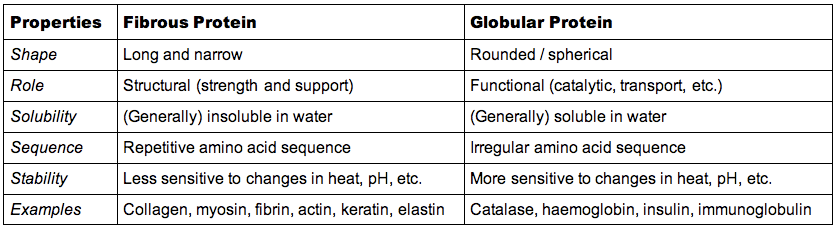
- Contrast the structure of globular proteins with the structure of fibrous proteins.
- Describe the structure of membrane bound globular proteins.
2.4.U5: A protein may consist of a single polypeptide or more than one polypeptide linked together.
- Outline the structure and function of an example protein composed of two or more polypeptides linked together.
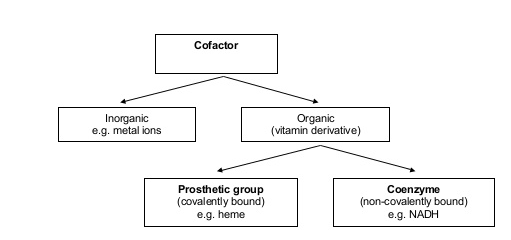
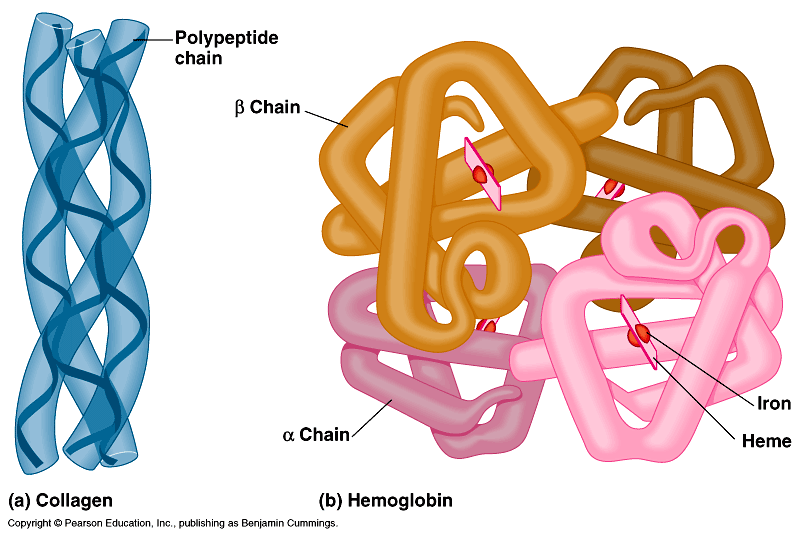
7.3.10: The quaternary structure exists in proteins with more than one polypeptide chain (may also involve the binding of a prosthetic group to form a conjugated protein)
- Outline the quaternary structure of protein folding.
- Describe the structure of a conjugated protein, including the prosthetic group.
2.4.U8: Every individual has a unique proteome.
- Define proteome.
- Contrast proteome with genome.
2.4.U7: Living organisms synthesize many different proteins with a wide range of functions.
- Contrast the generalized function of globular proteins with generalized function of fibrous proteins.
- List functions of proteins in a cell or organism.
- Describe the function of enzyme proteins.
- Describe the function of hormone proteins.
- Describe the function of immunoglobulin proteins.
- Describe the function of pigment proteins.
- Describe the function of structural proteins.
- Describe the function of contraction proteins.
- Describe the function of storage proteins.
- Describe the function of transport proteins.
- Describe the function of receptor proteins.
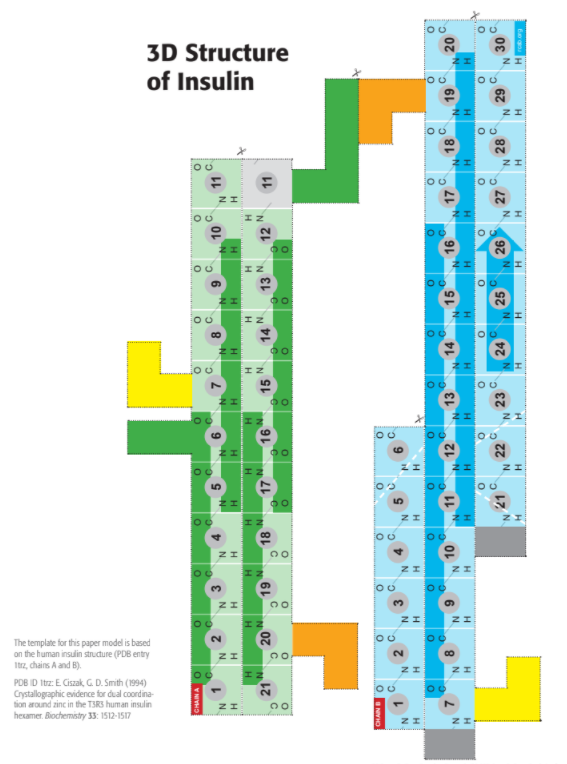
2.4.A1: Rubisco, insulin, immunoglobulins, rhodopsin, collagen and spider silk as examples of the range of protein functions.
- State the general function of each of the following proteins:
- Helicase
- Gyrase
- Primase
- DNA polymerase III
- DNA polymerase I
- Ligase
- RNA polymerase
- Amino-acyl tRNA synthetase
- Taq Polymerase
- Rubisco
- Restriction enzymes (endonucleases)
- ATP synthase
- Carboxylase
- Amylase
- Lipase
- Protease (pepsin and trypsin)
- Insulin
- Glucagon
- Thyroxin
- Leptin
- Melatonin
- Epinephrine
- FSH
- LH
- HCG
- ADH
- Oxytocin
- Growth hormone
- Prolactin
- Auxin
- Rhodopsin
- chlorophyll
- Sodium-potassium pump
- Potassium channel
- Hemoglobin
- Fetal hemoglobin
- Myoglobin
- Ferritin
- Silk
- Collagen
- Histones
- Single stranded binding proteins
- Actin
- Myosin
- Tropomyosin
- troponin
- Immunoglobulin
- antibodies
- Fibrinogen
- Fibrin
- Thrombin
- Histamine
Amino Acids
Amino acids are the basic units from which proteins are made. Plants can manufacture all of the amino acids they require from simpler molecules, but animals must obtain a number of ready-made amino acids (called essential amino acids) from their diet. All other amino acids can be constructed from these essential amino acids. The order in which the different amino acids are linked together to form proteins is controlled by genes on the DNA.
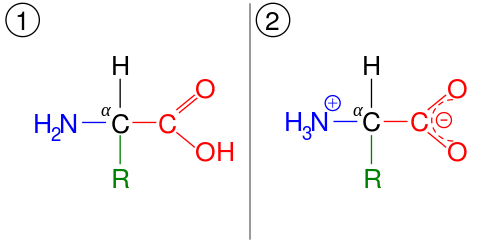
Structure of Amino Acids
There are over 150 amino acids found in cells, but only 20 occur commonly in proteins. The remaining, non-protein amino acids have specialized roles as intermediates in metabolic reactions, or as neurotransmitters and hormones. All amino acids have a common structure (see right). The only difference between the different types lies with the ‘R’ group in the general formula (‘R’ stands for radical). This group is variable, which means that it is different in each kind of amino acid. | General Structure of an Amino Acid
|
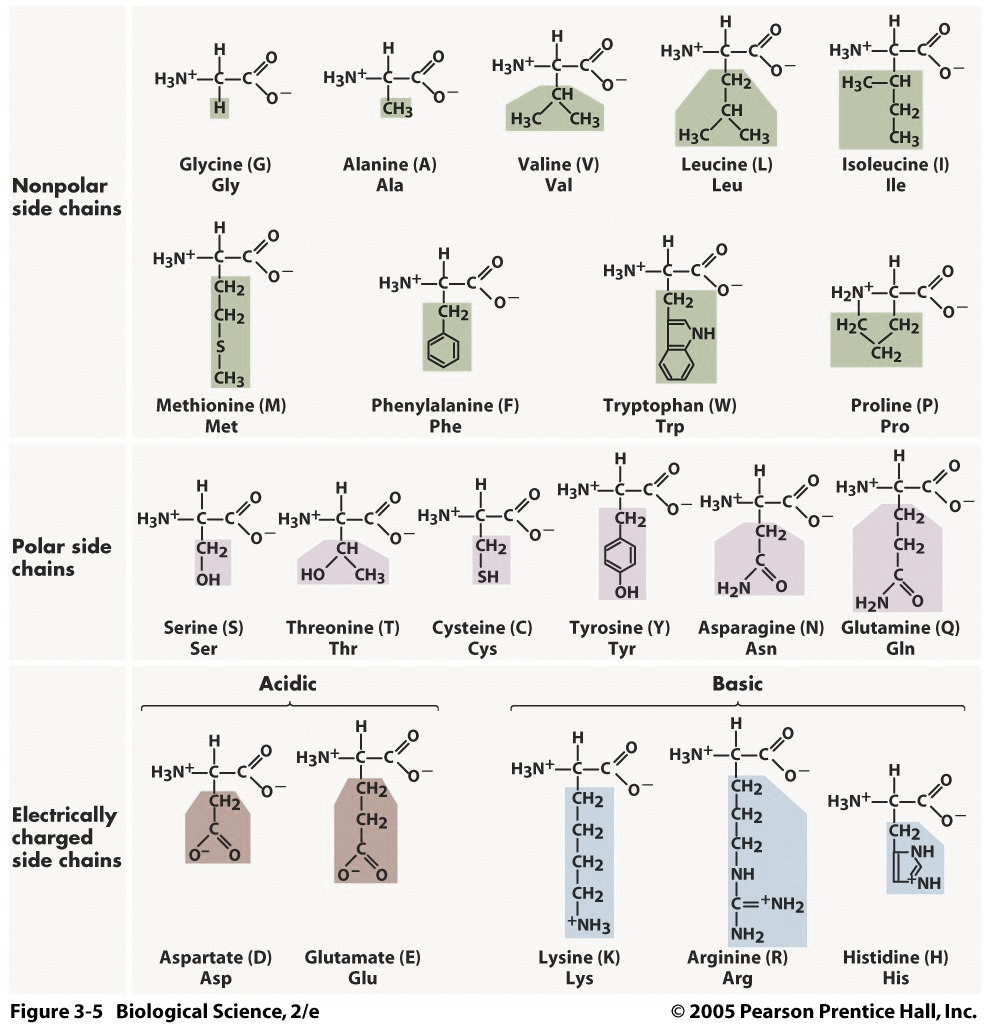
How to tell the properties of an amino acid:
- It is an amino acid? Do you see an amine group, a carboxyl group and an alpha carbon?
- Find the R group. Do you see an oxygen atom?
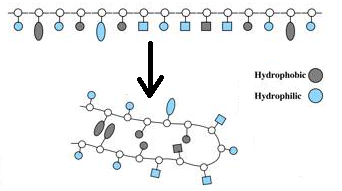
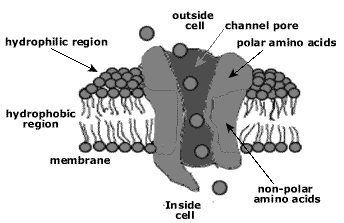
- NO…the amino acid is a NON-POLAR hydrocarbon (electrons are shared equally between the carbons and hydrogen). The amino acid will be hydrophobic (no attraction of water) and will most likely fold into the interior of the amino acid. If embedded in the cell membrane, the amino acid will probably be found in the hydrophobic interior.
- YES… ask yourself, do you see a CHARGE (+ or – sign) next to any of the atoms of the R group?
- NO … the amino acid is POLAR (electrons are shared equally between the oxygen and hydrogen, just like in water). The amino acid will be hydrophilic (capable of forming a H bond with water). The amino acid will also be capable of hydrogen bonding with other polar amino acids. The amino acid will most likely fold into the exterior of the amino acid. If embedded in the cell membrane, the amino acid will probably be found in the hydrophilic exterior.
- YES… the amino acid is an ION (has fully gained or lost an electron). These amino acids will be hydrophilic (capable of forming an H bond with water). The amino acid will also be capable of forming an ionic bond with oppositely charged ionic amino acids. The charged amino acids will most likely fold into the exterior of the protein. If embedded in the cell membrane, the amino acid will probably be found in the hydrophilic exterior.
Amino Acids in Nutrition

Lack of essential amino acids 🡪
Body can’t make the proteins that it needs 🡪
“Protein malnutrition”
From Gene to Protein:







Peptide Bond Formation
Peptide bonds link many amino acids together in long polymers called polypeptide chains. The ribosome catalyzes peptide bond formation between amino acids during translation elongation. The ribosome can make peptide bonds between any pair of amino acids, so any sequence of amino acids is possible.
Structure Name | Number of Amino Acids in the Peptide | The Number of Possible Amino Acid Sequences | Number of Peptide Bonds in the Polypeptide |
Amino Acid | 1 | 201 |
|
|
Dipeptide | 2 | 202 | 400 |
|
Oligopeptides (3-20 amino acids) | 3 |
| 8,000 |
|
4 |
|
|
|
| 206 | 64,000,000 |
|
18 |
|
|
|
Polypeptides (20+ amino acids) | 94 |
|
|
|
137 |
|
|
|
6347 |
|
|
|
Levels of Protein Folding
Level | Part of amino acid involved | Type of bond(s) | Diagram |
Primary |
|
| 
|
Secondary |
|
| 
|
Tertiary |
|
| 
|
Quaternary |
|
| 
|
Protein Cofactors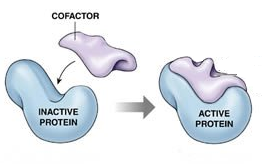
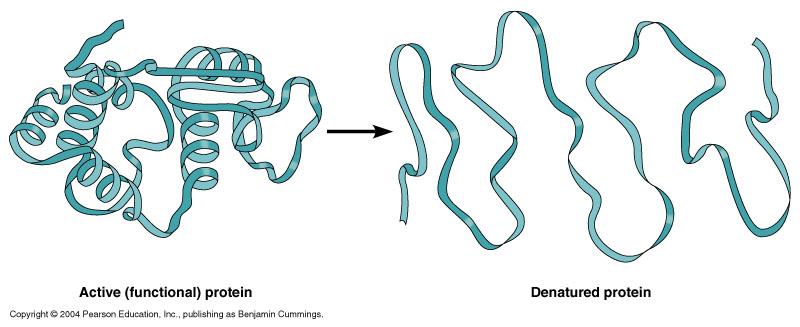
Protein Denaturation
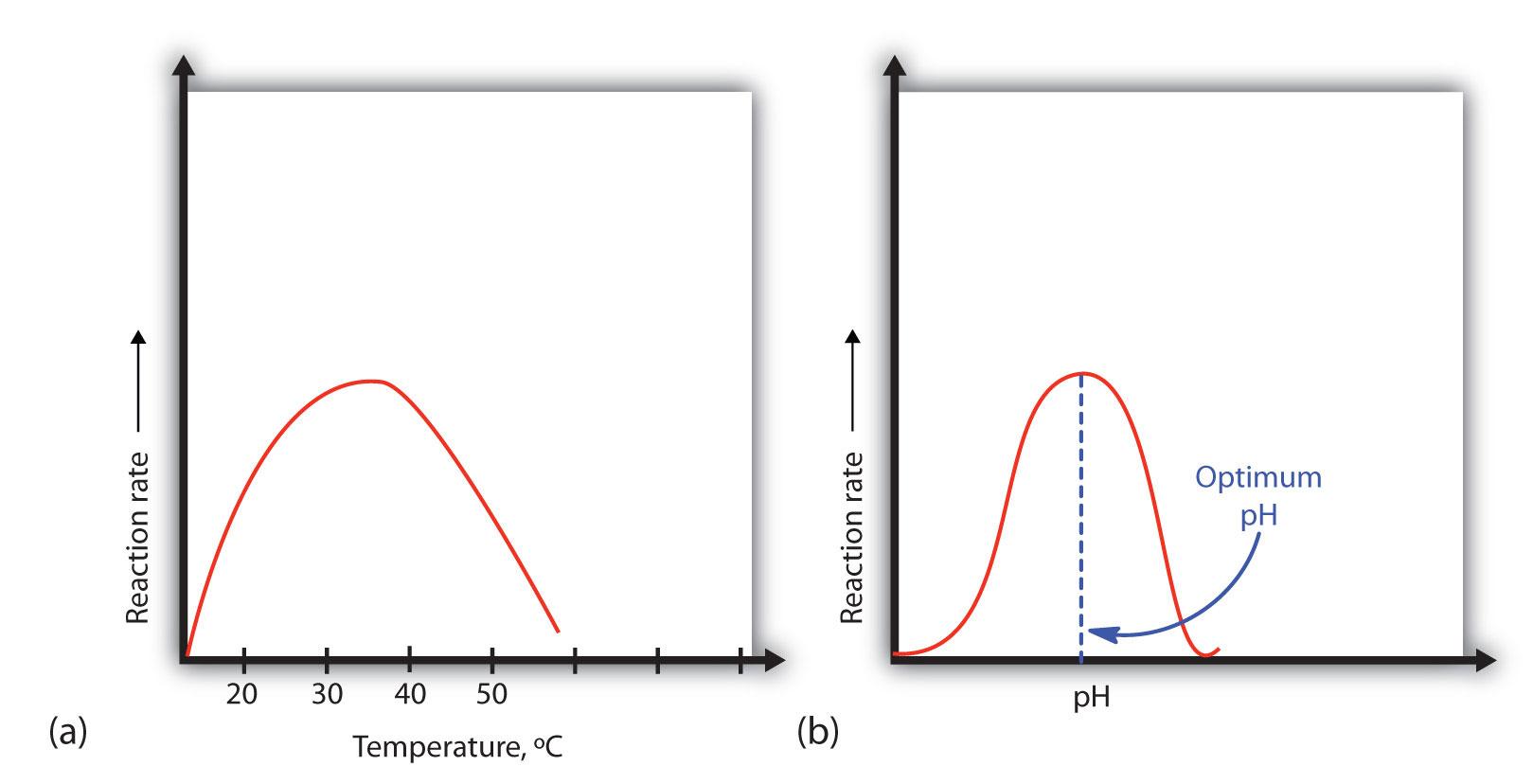
Classes of Proteins
PROTEIN FUNCTIONS
PROTEIN FUNCTION | DESCRIPTION OF FUNCTION | EXAMPLE PROTEINS | GLOBULAR OR FIBROUS |
Enzyme |
|
|
|
Hormone |
|
|
|
Immune Defense |
|
|
|
Pigment |
|
|
|
Structure |
|
|
|
Contraction |
|
|
|
Storage |
|
|
|
Transport |
|
|
|
Receptor |
|
|
|